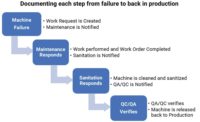
Sanitation software evolves, becomes paperless
Sanitation frequency, protocols, allergens are top of mind for snack and bakery companies




Food industry software solutions are evolving to include sanitation protocols, as paperless solutions are increasingly desired. In addition, allergen concerns factor into operational considerations, and software can help keep track of those concerns, too.
Scheduling software
“For bakery and snack food production facilities, there are several factors to consider when determining a frequency of sanitation,” says Evan Reyes, director of sales, sanitary division, Goodway Technologies, Stamford, CT. Production dynamics to consider include the microbial risk profile and water activity of the product and ingredients, the shelf life of the product, risk for microbial growth in different parts of the production process, the need to changeover between products, how long production equipment can run without failure, pest life cycles, and if a dry environment can be maintained during production and also during sanitation, he says.
“All of these risks should be considered when determining a frequency for sanitation in every facility. Some examples of how much the cleaning frequency can vary per product—sweet good bakeries with a higher risk profile may perform sanitation many times per week on night shift, while fresh bread and bun bakery sanitation is completed twice per week (or at least once a week during busy summer months), and sanitation in dry cookie/cracker environments can be stretched out to every 28 days if a dry environment is maintained and the risk profile of the particular product is low,” Reyes advises.
Robert Burgh, president, Nexcor Food Safety Technologies, Atlanta, says that its KLEANZ Family of Services encompasses unique solutions where they work with clients to incorporate their methods of operation and food safety into the KLEANZ system, not the other way around. “We don’t force clients to conform to our system,” he says. “It’s not an ‘out of the box’ solution.”
Burgh emphasizes that the importance of having a reliable food safety system is astronomical. “The risk of undeclared allergens can come to fruition monetarily, pose harm to customers, and ruin a brand’s identity.”
Burgh says that the KLEANZ Family of Services offers proven data collection efforts to ensure that client schedules are incorporated into, and optimized in, the system. “Daily work, interval work, non-FSMA zone cleaning, and everything in between is precisely aggregated into the system with alerting features and automatic completion reporting. The system prevents scheduling redundant cleaning by setting priority to the task involved. For example, you wouldn’t clean equipment before you clean everything above the equipment. KLEANZ closes the gaps so that important work can’t be missed, and all work has appropriate documentation behind it.”
Stephen Dombroski, director, consumer, food & beverage markets, QAD, Inc., Santa Barbara, CA, says that QAD has functionality to help all types of food and beverage companies to operate their plants efficiently, by keeping equipment running at the lowest costs. “Plant maintenance and operations supports preventative and predictive maintenance, as well as planned and unplanned work needed to keep equipment reliable and up and running. In addition, all safety rules and initiatives to prevent contaminants can be followed to ensure proper cleanouts.”
Production scheduling workbench provides schedulers with complete visibility into all key factors important to planning and scheduling decisions, including capacity and materials availability, Dombroski comments. “Schedulers can manage production sequences to minimize changeovers, and to ensure that proper types and levels of changeovers and clean-outs are scheduled after certain products, due to allergen or composition requirements requiring complete system flush.”
Protocols and safeguards
So what happens if protocol is breeched, and should sanitation software have safeguards in place?
“There are two types of scenarios that can be considered in this case,” says Dombroski. “First, planning and scheduling. The system can be configured as such that production of certain products will not be scheduled unless a washout/changeover was scheduled prior to the production run. The second is if someone on the factory floor executes a production run without having performed the associated cleanup. This can be configured through production execution functionality. Ultimately however, if this would occur, it would be a human error. But all the necessary alerts are in the software to plan for all necessary regulatory requirements governing maintaining allergen control.”
Burgh says that the KLEANZ Family of Services has several features that notify management of breached protocol, incomplete work, missing training, etc. “These features are completely configurable and can be tied in with other facility operating systems through KLEANZ Connect. Notifications and alerts can be scheduled as reoccurring or one-off SMS messages, emails, automatic reports, and more. They can also be escalating, moving up the chain of command according to parameters like severity.”
For safeguards, Dombroski says that QAD offers several product solutions to insure that safe protocols are met in terms of production scheduling, so that products requiring changeovers are maintained in sequence and through quality management. “QAD offers a complete quality management system that helps maintain all levels of product safety, security as well as operational safety and quality. Incorporating quality management into every stage of the design and production process prevents problems from reaching customers. A preventative approach saves money and enhances your company’s reputation.”
QAD Product Genealogy provides centralized visibility for tracking critical quality issues during the manufacturing and distribution processes, Dombroski says. “It simplifies tracing the origination of raw or component materials, product composition, shipping destinations and material and product compliance with quality specifications. The solution enables manufacturers to flexibly configure item dimensions to track industry and customer specific requirements and mandates.”
There are three primary components to the QAD Product Genealogy solution, Dombroski explains:
- QAD Serialization provides inventory identification and full track and trace of each event by unique identifiers, whether the inventory consists of packaging units or specific purchased, produced or shipped item units. It enables tracking and tracing of both lot and serial item units using high volume serial numbers tightly integrated with packaging handling techniques.
- QAD Item Attributes and Quality Control allows you to assign specific attributes to items such as quality specifications, use-by dates or country of origin to ensure that you meet the quality specifications required by your customers, industry or governmental regulators.
- QAD Lot Trace Workbench extends track and trace capabilities and provides visibility of current and historical lot-controlled inventory to support returns, recalls and audits. It enables forward and backward tracing, whether initiating a trace from raw material or from shipment. When product quality issues arise, Lot Trace Workbench enables you to trace the root cause of the issue quickly and easily.
“These capabilities help manufacturers ensure that all items meet product quality standards and specifications from the point of receipt, through production, to customer shipment,” says Dombroski.
Burgh says that the KLEANZ Family of Services safeguards all aspects of food safety, including elements that intend to have an impact on food safety. “The system maintains a complete audit trail and compiles data into useful information. This information, for example, can be automatically presented to corporate, decision makers on a weekly basis. Transparency companywide is a critical safeguard against risk.”
KLEANZ also ensures accuracy with sign-off features. Management can opt to sign-off that work is done to specification before it’s considered complete, says Burgh. “KLEANZ incorporates document attachment capability to scheduled work. Managers can attach instructional documents and images to tasks. This mitigates the risk of confusion and the possibility of tasks being completed inaccurately.”
Full vs. partial cleandown
Companies making allergen-free snacks must be especially careful about sanitation and when they perform cleandowns.
Changing over from a product containing an allergen to a non-allergen product on the same production line automatically dictates a full sanitation, with extra care taken for sanitation to ensure food contact surfaces are 100 percent visually cleaned and food contact surfaces pass allergen test kit swabs, says Reyes.
“The later the allergen is introduced in the production process, the less the equipment has to be thoroughly cleaned and swabbed to an allergen removal level. This is why it is so challenging—and, in many cases, impractical—to do a gluten/wheat changeover in a bakery, or to changeover from milk to dark chocolate in a confectionery facility, as wheat and milk touch every piece of production equipment in these facilities,” Reyes notes.
When a product includes ingredients that have higher sanitation issues—stickiness, or higher water activity—sanitation will need to be carried out more frequently, Reyes comments. “There is risk of these ingredients introducing additional microbial risk, as well as potentially stopping production. It is important to consider the growth rate of microorganisms within these ingredients and decide on a sanitation frequency that prevents any associated risks.”
It is fine to do a partial sanitation instead of the facility’s normal sanitation during quick product changeovers that don’t involve an allergen (taste, color, smell), or less-stringent mid-shift cleanings, says Reyes. Sanitation procedures vary greatly due to the varying risks that can come into play, he notes, as well as the stated goal of sanitation—visual clean, allergen clean, micro clean, etc.
Reyes recommends working very closely with quality and food safety teams at the company to understand the risks within each facility and production line. “Determine the goal for sanitation in each application, understand the sanitation procedures and validation methods used to ensure sanitation is effective, and then allow snack producers and bakers to more easily execute the resultant sanitation procedures consistently and effectively.”
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!