Home » FDA
Articles Tagged with ''FDA''
Sweeteners help snack and bakery manufacturers offer better-for-you products
Strategies for sweetener use factor in several variables, including 'added sugars,' better-for-you positioning, flavor, and natural and clean-label requirements.
January 16, 2017
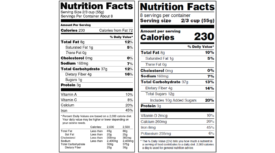
KIND publishes added sugar content of products online
The publication comes two years before the deadline set by the FDA.
August 10, 2016
FDA's Added sugars labeling leads to added complexities
Scientists waffle on whether added sugars are really any different.
July 26, 2016
FDA reverses ruling, allows KIND to label bars ‘healthy’
Company says ruling highlights the inconsistency in FDA's regulation on how the label can be used.
May 11, 2016
Snack on the latest trends, news, and developments!
Stay in the know with Snack Food & Wholesale Bakery, the premier source of information for snack, bakery, and confectionery professionals.
JOIN TODAY!Copyright ©2024. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing